Understanding role of Ribonuclease inhibitor (RNH1) in Myelopoiesis and Myeloid Malignancies
Allam group Prof. Dr. phil. nat. Ramanjaneyulu Allam
Our previous investigations have unveiled a crucial function of Ribonuclease inhibitor (RNH1) in regulating homeostatic hematopoiesis. In the absence of RNH1, the balance in hematopoiesis was skewed significantly to favour myelopoiesis at the expense of lymphoid and erythroid lineage cells. It has also been observed from our in-vivo mice studies that the increase in myelopoiesis however, did not culminate in a leukemic transformation.
The ongoing project is aimed at elucidating the mechanism behind the RNH1 mediated exacerbation of myelopoiesis under homeostatic conditions and the implications of genetically modifying RNH1 expression in myeloid malignancies. For this, we are utilizing AML in-vitro (cell lines) and in-vivo (mouse) models along with AML patient derived cells.
Our research so far has highlighted an involvement of RNH1 in the AML pathophysiology and ongoing studies are attempted at discerning a therapeutic potential of targeting RNH1 in myeloid malignancies.
Next-generation CAR T cells for pediatric solid tumors
Bernasconi Furtwängler group Prof. Dr. Michele Bernasconi
We aim to improve existing therapies for pediatric solid tumors and to develop more effective and less toxic treatment strategies, with a particular focus on rhabdomyosarcoma and Wilms tumor.
Pediatric sarcomas account for approximately 15% of childhood cancers, and relapse rates remain high with an extremely poor prognosis. Rhabdomyosarcoma (RMS) is the most common soft-tissue sarcoma in children. Two major prognostic subgroups are recognized: fusion-negative (FN) RMS, present in about 55% of patients and associated with a five-year event-free survival of ~70%, and fusion-positive (FP) RMS, present in ~20% of patients with a markedly lower five-year event-free survival of ~23%. Wilms tumor (WT), or nephroblastoma, is the most frequent renal tumor in childhood. While curative therapy is achievable in the majority of cases, patients with high-risk tumors (blastemal or anaplastic histology) have significantly poorer survival. Moreover, current treatment regimens are associated with relevant long-term sequelae, including secondary neoplasms, cardiac insufficiency, infertility, and renal impairment.
CAR T cells are genetically engineered T lymphocytes expressing chimeric antigen receptors (CARs). CAR T-cell therapy represents one of the most promising approaches for relapsed or otherwise refractory cancers.
Since 2018, our laboratory has focused on advancing this personalized immunotherapy by strengthening the patient’s immune capacity to recognize and attack tumors. Using proteomics, we investigate the surfaceome to identify novel targets for CAR T-cell therapy. We then perform in vitro and in vivo studies to enhance CAR T-cell activity and therapeutic efficacy.
Perspectives of Targeted Radionuclide Imaging and Therapy of Fibroblast Activation Protein (FAP) in Cancer
Gourni group PD Dr. Eleni Gourni
Tumors develop within a complex microenvironment consisted of diverse cell types surrounded by a matrix rich of proteins, termed tumor stroma. Stroma includes immune cells, fibroblasts and vascular enothelial cells.
Cancer cells rely on extensive support from the stroma to survive, proliferate and invade, thus making stroma an important potential target for anti-cancer therapy. Targeting elements of stroma, may be a useful therapeutic strategy to prevent tumor growth and progression. One of those elements is the fibroblast activation protein (FAP) which is overexpressed on activated fibroblasts on several tumors types.
The current project aims at designing and evaluating novel FAP-specific inhibitors for the generation of radiotracers with the potential to be used for the diagnosis and treatment of FAP-positive tumors. The novel radiotracers are thoroughly investigated in vitro and in vivo using cell lines and xenografted tumor models to understand their binding properties and their in vivo performance.
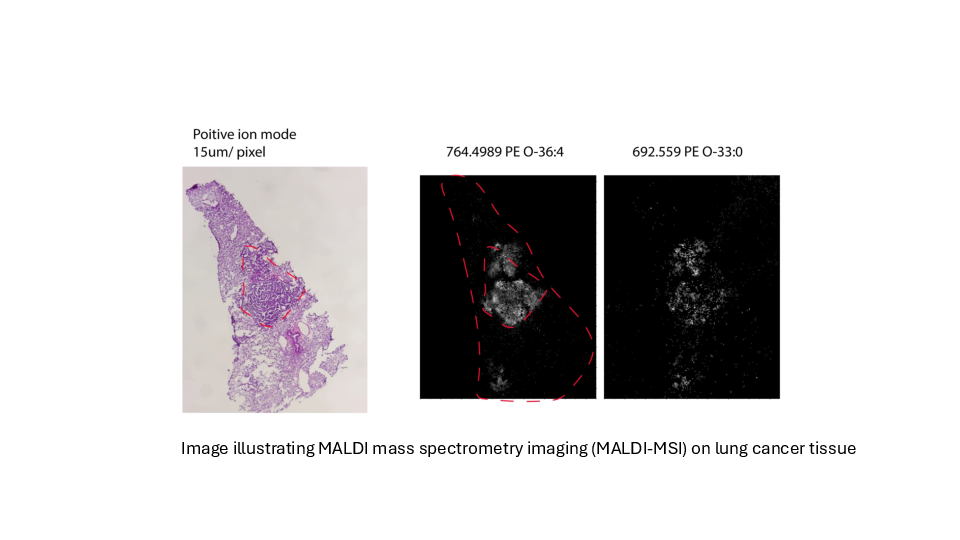
Determing tumor lipid heterogeneity in lung cancer
Konstantinidou group PD. Dr. Georgia Konstantinidou, PhD
Lung cancer is the most common cause of cancer-related deaths worldwide. Tumor-associated mutations of KRAS occur in approximately 30% of non-small cell lung cancer, the most common form of lung cancer.
KRAS mutations are associated with aggressive, metastatic, and treatment-resistant cancers in both humans and mouse models. Mutant KRAS drives a complex network of lipid metabolic rearrangements to help cancer cells adapt to hypoxia and ensure their survival.
We plan to determine the transcriptomic and lipidomic changes that occur in lung tumors during cancer progression and therapy resistance and assess their functional significance, accounting for tumor-to-tumor heterogeneity while preserving the spatial organization of cancer cell populations within the tumor microenvironment.
Dissecting the role of tumor cell heterogeneity in Pancreatic Neuroendocrine Tumor progression
Group Marinoni, Perren, Sadowski PD Dr. Ilaria Marinoni, PhD, Prof. Dr. med. Aurel Perren, PD Dr. Martin Sadowski, PhD
Cancer is a dynamic disease; genetic and epigenetic alterations drive intra-tumoral cell heterogeneity, resulting in the selection of aggressive cell populations capable of driving progression and ultimately metastasis.
Pancreatic neuroendocrine tumours (PanNETs) are tumours that arise from the islets of Langerhans. They exhibit intra-tumoral cell heterogeneity, but it is unclear how this evolves during tumour development and how it contributes to progression.
Our previous data suggest that epigenetic changes are the major drivers of progression and cell heterogeneity in PanNETs. By integrating epigenetic and transcriptomic profiles, we found that cell dedifferentiation and metabolic changes characterise the progression from small PanNETs to more advanced ones.
We are currently investigating the evolution of intra-tumoral heterogeneity of PanNETs through space and time. Specific cell subpopulations identified as driving progression could then be targeted to stop metastasis formation. The identification of targetable pathways that impair metastasis formation will provide a rationale for new treatments.
Targeting cellular metabolism to augment cancer therapy
Marti Group PD Dr. med. Thomas Marti
The aim of this project is to investigate how the nucleotide/lactate metabolism and the DNA damage response machinery are associated with the tumor initiating capacity, the chemotherapy response, and the metastatic capacity of lung and mesothelioma cancer stem cells. In addition, we are exploiting treatment induced cellular adaptations as novel targets for cancer therapy.
Oncogenic signaling via receptor tyrosine kinases in crosstalk with DNA damage repair
Medová Group PD Dr. med. Michaela Medová
Tyrosine kinase receptors activate a wide range of different cellular signaling pathways. Physiologically, intact signaling via the MET receptor is indispensable in embryonic development and tissue homeostasis. At the same time, MET dysregulation promotes features clearly associated with tumor growth and progression such as uncontrolled proliferation, angiogenesis, local invasion, and systemic dissemination.
Accumulating data suggest that MET signaling may also protect tumor cells from DNA damage, hence relating its aberrant activity to resistance to DNA-damaging agents routinely used in cancer treatment.
We have identified a previously unreported phosphorylation site on MET, which can be recognized by DNA damage master kinases and is involved not only in cellular responses towards DNA damage, but also in metastatic processes, cancer cell migration, and anchorage-independent growth.
This project aims at dissecting the nature, function, and regulation of this phosphorylation site in oncogenic signaling of the receptor.
Myeloid Malignancies
Meyer group Prof. Dr. med. Sara C. Meyer
Myeloproliferative neoplasms (MPN) are chronic leukemias characterized by constitutive activation of JAK2 tyrosine kinase signaling. Clinical JAK2 inhibitors bring benefits for patients, but have limited disease-modifying activity. Allogeneic hematopoietic cell transplantation is the only curative treatment to date.
The Meyer lab has a specific interest in the oncogenic signaling driving MPN. We have demonstrated that activation of the MAPK pathway with MEK1/2 and ERK1/2 kinases, which is involved in several cancers, limits JAK2 inhibitor therapy and needs to be adressed to enhance efficacy (Stivala, JCI 2019; Brkic, Leukemia 2021). These findings have translated to a clinical study (Adore, NCT04097821).
Our lab is investigating mechanisms of resistance, which mediate loss of response to clinical JAK2 inhibitors, and approaches to overcome resistance. Notably, we are involved in the characterization of novel types of JAK2 inhibitors incl. type II JAK2 inhibitors currently in development towards clinical studies (Meyer, Cancer Cell 2015; Codilupi, CCR 2024).
The interaction between immune cells and leukemia/cancer stem cells
Ochsenbein group Prof. Adrian Ochsenbein, MD
Our research unit examines the interaction between immune cells and cancer stem cells with a focus on leukemia stem cells. Cancer stem cells are resistant to most of the currently available drugs and are responsible for relapse after successful chemotherapy.
We use state of the art techniques to analyse the molecular interactions between immune cells and cancer stem cells in murine models and in clinical samples from cancer patients.
The aim is to develop improved immunotherapies that specifically target cancer stem cells for different types of cancer, especially in hemato-oncological diseases such as leukemia and multiple myeloma. These novel durgs are tested in in preclinical models and in clinical phase 1 and 2 studies.
Genetic variants of immune checkpoint regulators as response biomarkers in B-cell lymphoma treated with CAR-T cell therapy
Pabst & Seipel group Prof. Dr. med. Thomas Pabst, PD Dr. Katja Seipel
Starting in 2020 CAR T-cell therapy has been applied in R/R B-cell lymphoma at Inselspital Bern. Biomarkers of response were evaluated in retrospective analysis in order to predict treatment outcome and improve CAR-T cell therapy.
Targeting Metabolic Supercomplexes in Therapy-Resistant Prostate Cancer
Pandey group Prof. Dr. phil. Amit V. Pandey
Castration-resistant prostate cancer (CRPC) represents a lethal stage of the disease, primarily driven by the tumor's ability to overcome therapy through the synthesis of its own androgens.
Our research has advanced beyond studying single enzymes to investigate their higher-order organization into what we term "metabolic supercomplexes" or "metabolons."
Our central hypothesis is that key enzymes in androgen production, such as CYP17A1, AKR1C3, and STS, do not function in isolation. Instead, they form organized, multi-protein complexes at the interface of cellular compartments, like the endoplasmic reticulum and the cytosol. These supercomplexes act as hyper-efficient production lines, utilizing a mechanism called "substrate channeling" to rapidly convert precursors into potent androgens that fuel cancer growth.
This model provides a powerful new explanation for the robust resistance observed against drugs like abiraterone. Our current work focuses on characterizing the structure and function of these supercomplexes. The ultimate goal is to develop innovative therapeutic strategies that not only inhibit key enzymes but also disrupt the crucial protein-protein interactions that hold these metabolic machines together, potentially using novel small molecules or advanced nanoparticle-based delivery systems.
Leukemia stem cells and the bone marrow microenvironment
Riether group Prof. Carsten Riether, PhD
The bone marrow (BM) microenvironment is a unique cellular architecture which crucially regulates self-renewal and differentiation potential of hematopoietic stem and progenitor cells through cell-cell interaction or the release of soluble mediators.
These evolutionary conserved processes that evolved to protect normal hematopoietic stem cells from elimination and to regulate demand-adapted responses during inflammation are frequently hijacked in cancer and leukemia.
The goal of our research is to understand the molecular and cellular mechanisms how different components of the BM microenvironment such as immune cells and stromal cells affect disease-initiating and -maintaining leukemia stem cells (LSCs) and protect them from immune-mediated elimination.
We take advantage of state-of-the art technologies, well-established chronic and acute myeloid leukemia mouse and patient-derived xenograft models in order strengthen our understanding on LSCs and to translate our findings into human disease.
Cancer cell motility supported by oncogene induced autophagy
Tschan group Prof. Dr. phil. nat. Mario Tschan
We discovered an oncogenic splice variant of the tumor suppressor and transcription factor DMTF1 active in the p53 pathway. We found that this splice variant, DMTF1β, promotes breast cancer cell motility by activating autophagy.
We are currently unravelling mechanisms how DMTF1β is regulated and how it promotes cancer cell motility by activating autophagy.
Our aim is to identify tumor types and cellular conditions where common cancer therapies in combination with autophagy inhibition is beneficial to block migration.
Nonsense-mediated mRNA decay, the achilles heel of recurrent glioblastoma?
Vassella group Prof. Dr. Erik Vassella
We conducted CRISPR/CAS interference library screens and identified SMG1, implicated in an evolutionarily conserved RNA quality control pathway - the nonsense-mediated mRNA decay (NMD) pathway. NMD leads to the degradation of transcripts containing premature stop codons, often occurring after temozolomide treatment.
NMD may influence the mechanisms employed by tumour cells to repair DNA damage caused by temozolomide treatment. Hence, we hypothesise that the enhanced temozolomide response is due to reduced DNA repair capacity in SMG1-attenuated glioblastoma cells.
The aim of this work is to further investigate the mechanisms leading to an enhanced temozolomide response in glioblastoma cells with attenuated SMG1. Since NMD efficiently suppresses truncated proteins, which are highly immunogenic, we hypothesise that SMG1 inhibition in temozolomide-resistant, recurrent glioblastoma may elicit tumour inflammation. Hence, we expect that NMD improves the treatment response to immune checkpoint inhibitors. This work is currently supported by the Swiss Cancer League.