Next-generation CAR T cells for pediatric solid tumors
Bernasconi Furtwängler group Prof. Dr. Michele Bernasconi
We aim to improve existing therapies for pediatric solid tumors and to develop more effective and less toxic treatment strategies, with a particular focus on rhabdomyosarcoma and Wilms tumor.
Pediatric sarcomas account for approximately 15% of childhood cancers, and relapse rates remain high with an extremely poor prognosis. Rhabdomyosarcoma (RMS) is the most common soft-tissue sarcoma in children. Two major prognostic subgroups are recognized: fusion-negative (FN) RMS, present in about 55% of patients and associated with a five-year event-free survival of ~70%, and fusion-positive (FP) RMS, present in ~20% of patients with a markedly lower five-year event-free survival of ~23%. Wilms tumor (WT), or nephroblastoma, is the most frequent renal tumor in childhood. While curative therapy is achievable in the majority of cases, patients with high-risk tumors (blastemal or anaplastic histology) have significantly poorer survival. Moreover, current treatment regimens are associated with relevant long-term sequelae, including secondary neoplasms, cardiac insufficiency, infertility, and renal impairment.
CAR T cells are genetically engineered T lymphocytes expressing chimeric antigen receptors (CARs). CAR T-cell therapy represents one of the most promising approaches for relapsed or otherwise refractory cancers.
Since 2018, our laboratory has focused on advancing this personalized immunotherapy by strengthening the patient’s immune capacity to recognize and attack tumors. Using proteomics, we investigate the surfaceome to identify novel targets for CAR T-cell therapy. We then perform in vitro and in vivo studies to enhance CAR T-cell activity and therapeutic efficacy.
A personalized multi-omics discovery and validation platform for recurrent head and neck squamous cell carcinoma (POLARES)
Giger group Prof. Dr. med. Roland Giger
Diagnostic and therapeutic developments in recent years have improved the prognosis for patients with head and neck squamous cell carcinoma (HNSCC). Despite these developments, a significant proportion of patients relapse after an initial response to standard treatment. Salvage treatment options are limited, and personalized treatment approaches that consider the genomic/epigenetic landscape of the tumor are lacking.
The goal of this research is to establish a center of excellence in HNSCC that bridges the gap between genomic analysis and translation of findings into clinical trials. By establishing a multi-omics discovery and validation platform under the umbrella of the University Comprehensive Cancer Center Inselspital (UCI), this consortium (ORL, Head and Neck Surgery; Medical Oncology; Radiation-Oncology) aims to determine how alterations at the genomic and epigenetic level regulate carcinogenesis, treatment response and resistance in HNSCC and thereby identify novel mechanisms to target tumor relapse.
On behalf of the Consortium: Prof. Dr. Roland Giger (Lead), Otorhinolaryngology, Head and Neck Surgery; PD Dr. Olgun Eliçin, Radio-Oncology; Dr. Simon Häfliger, Medical Oncology; PD Dr. Michaela Medová, Radio-Oncology, DBMR; Prof. Dr. Carsten Riether, Medical Oncology, DBMR; Dr. Daniel H. Schanne, Radio-Oncology
Perspectives of Targeted Radionuclide Imaging and Therapy of Fibroblast Activation Protein (FAP) in Cancer
Gourni group PD Dr. Eleni Gourni
Tumors develop within a complex microenvironment consisted of diverse cell types surrounded by a matrix rich of proteins, termed tumor stroma. Stroma includes immune cells, fibroblasts and vascular enothelial cells.
Cancer cells rely on extensive support from the stroma to survive, proliferate and invade, thus making stroma an important potential target for anti-cancer therapy. Targeting elements of stroma, may be a useful therapeutic strategy to prevent tumor growth and progression. One of those elements is the fibroblast activation protein (FAP) which is overexpressed on activated fibroblasts on several tumors types.
The current project aims at designing and evaluating novel FAP-specific inhibitors for the generation of radiotracers with the potential to be used for the diagnosis and treatment of FAP-positive tumors. The novel radiotracers are thoroughly investigated in vitro and in vivo using cell lines and xenografted tumor models to understand their binding properties and their in vivo performance.
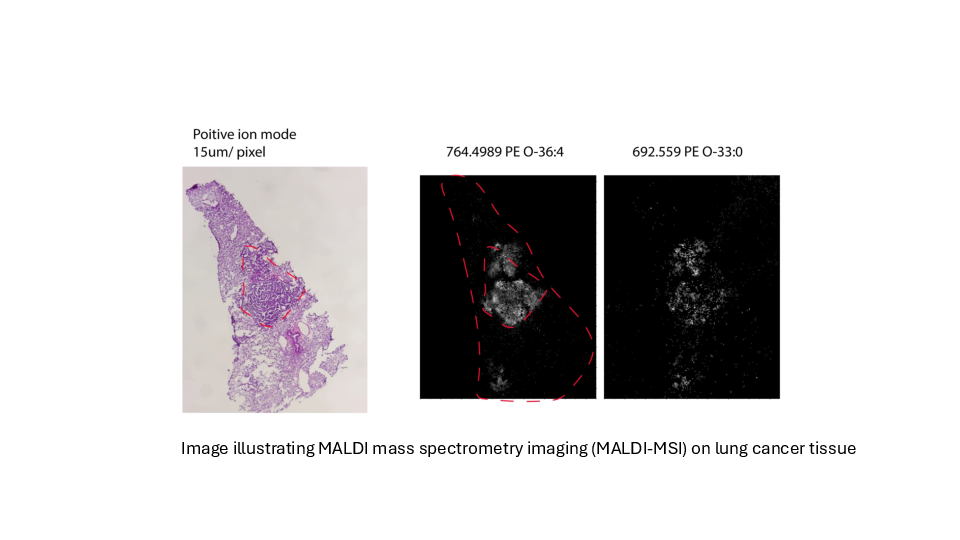
Determing tumor lipid heterogeneity in lung cancer
Guenat group Prof. Dr. Olivier T. Guenat
The Metastasis-on-Chip project aims to replicate the metastatic process, focusing specifically on extravasation and colony formation.
Our initial studies evaluate the metastatic potential of cancer cells based on their phenotypes, using the A549 non-small cell lung cancer (NSCLC) cell line, which exhibits distinct phenotypic variations. We discovered that paraclones, characterized by a mesenchymal phenotype, successfully extravasate, while holoclones, with an epithelial phenotype, do not. Additionally, paraclones demonstrated significantly greater migratory behavior compared to holoclones.
These findings provide valuable insights into the mechanisms of metastasis and lay the groundwork for further exploration of targeted therapies.
Determing tumor lipid heterogeneity in lung cancer
Konstantinidou group PD. Dr. Georgia Konstantinidou, PhD
Lung cancer is the most common cause of cancer-related deaths worldwide. Tumor-associated mutations of KRAS occur in approximately 30% of non-small cell lung cancer, the most common form of lung cancer.
KRAS mutations are associated with aggressive, metastatic, and treatment-resistant cancers in both humans and mouse models. Mutant KRAS drives a complex network of lipid metabolic rearrangements to help cancer cells adapt to hypoxia and ensure their survival.
We plan to determine the transcriptomic and lipidomic changes that occur in lung tumors during cancer progression and therapy resistance and assess their functional significance, accounting for tumor-to-tumor heterogeneity while preserving the spatial organization of cancer cell populations within the tumor microenvironment.
Targeting cellular metabolism to augment cancer therapy
Marti Group PD Dr. med. Thomas Marti
The aim of this project is to investigate how the nucleotide/lactate metabolism and the DNA damage response machinery are associated with the tumor initiating capacity, the chemotherapy response, and the metastatic capacity of lung and mesothelioma cancer stem cells. In addition, we are exploiting treatment induced cellular adaptations as novel targets for cancer therapy.
Oncogenic signaling via receptor tyrosine kinases in crosstalk with DNA damage repair
Medová Group PD Dr. med. Michaela Medová
Tyrosine kinase receptors activate a wide range of different cellular signaling pathways. Physiologically, intact signaling via the MET receptor is indispensable in embryonic development and tissue homeostasis. At the same time, MET dysregulation promotes features clearly associated with tumor growth and progression such as uncontrolled proliferation, angiogenesis, local invasion, and systemic dissemination.
Accumulating data suggest that MET signaling may also protect tumor cells from DNA damage, hence relating its aberrant activity to resistance to DNA-damaging agents routinely used in cancer treatment.
We have identified a previously unreported phosphorylation site on MET, which can be recognized by DNA damage master kinases and is involved not only in cellular responses towards DNA damage, but also in metastatic processes, cancer cell migration, and anchorage-independent growth.
This project aims at dissecting the nature, function, and regulation of this phosphorylation site in oncogenic signaling of the receptor.
Myeloid Malignancies
Meyer group Prof. Dr. med. Sara C. Meyer
Myeloproliferative neoplasms (MPN) are chronic leukemias characterized by constitutive activation of JAK2 tyrosine kinase signaling. Clinical JAK2 inhibitors bring benefits for patients, but have limited disease-modifying activity. Allogeneic hematopoietic cell transplantation is the only curative treatment to date.
The Meyer lab has a specific interest in the oncogenic signaling driving MPN. We have demonstrated that activation of the MAPK pathway with MEK1/2 and ERK1/2 kinases, which is involved in several cancers, limits JAK2 inhibitor therapy and needs to be adressed to enhance efficacy (Stivala, JCI 2019; Brkic, Leukemia 2021). These findings have translated to a clinical study (Adore, NCT04097821).
Our lab is investigating mechanisms of resistance, which mediate loss of response to clinical JAK2 inhibitors, and approaches to overcome resistance. Notably, we are involved in the characterization of novel types of JAK2 inhibitors incl. type II JAK2 inhibitors currently in development towards clinical studies (Meyer, Cancer Cell 2015; Codilupi, CCR 2024).
The interaction between immune cells and leukemia/cancer stem cells
Ochsenbein group Prof. Adrian Ochsenbein, MD
Our research unit examines the interaction between immune cells and cancer stem cells with a focus on leukemia stem cells. Cancer stem cells are resistant to most of the currently available drugs and are responsible for relapse after successful chemotherapy.
We use state of the art techniques to analyse the molecular interactions between immune cells and cancer stem cells in murine models and in clinical samples from cancer patients.
The aim is to develop improved immunotherapies that specifically target cancer stem cells for different types of cancer, especially in hemato-oncological diseases such as leukemia and multiple myeloma. These novel durgs are tested in in preclinical models and in clinical phase 1 and 2 studies.
Genetic variants of immune checkpoint regulators as response biomarkers in B-cell lymphoma treated with CAR-T cell therapy
Pabst & Seipel group Prof. Dr. med. Thomas Pabst, PD Dr. Katja Seipel
Starting in 2020 CAR T-cell therapy has been applied in R/R B-cell lymphoma at Inselspital Bern. Biomarkers of response were evaluated in retrospective analysis in order to predict treatment outcome and improve CAR-T cell therapy.
Leukemia stem cells and the bone marrow microenvironment
Riether group Prof. Carsten Riether, PhD
The bone marrow (BM) microenvironment is a unique cellular architecture which crucially regulates self-renewal and differentiation potential of hematopoietic stem and progenitor cells through cell-cell interaction or the release of soluble mediators.
These evolutionary conserved processes that evolved to protect normal hematopoietic stem cells from elimination and to regulate demand-adapted responses during inflammation are frequently hijacked in cancer and leukemia.
The goal of our research is to understand the molecular and cellular mechanisms how different components of the BM microenvironment such as immune cells and stromal cells affect disease-initiating and -maintaining leukemia stem cells (LSCs) and protect them from immune-mediated elimination.
We take advantage of state-of-the art technologies, well-established chronic and acute myeloid leukemia mouse and patient-derived xenograft models in order strengthen our understanding on LSCs and to translate our findings into human disease.
Towards understanding the role of the minor spliceosome in cancer
Rubin group Prof. Mark A. Rubin MD
Genes are composed of coding units (exons), interspersed with non-coding regions called introns. The process of protein production involves splicing together exons while removing introns from the mRNA molecule. Evolution has given rise to a cellular apparatus called the spliceosome, responsible for carrying out this splicing process.
Alternative splicing enables the generation of diverse protein isoforms from a single gene. Splicing is tightly regulated under normal physiological conditions.
Our recent findings indicate that cancer cells use a specialized spliceosome, the so-called minor spliceosome, to increase cancer relevant mRNAs. As such cancer hijacks the minor intron-splicing machinery to enhance the expression of transcripts containing minor introns. Proteins encoded by those genes have been shown to activate critical cell survival pathways such as cell cycle regulation and DNA repair.
Exploiting the reliance of cancer cells on minor intron-containing genes presents a novel therapeutic opportunity for targeting cancer. By inhibiting the minor spliceosome, we can selectively induce cell death in cancer cells while sparing healthy neighboring cells.
Nonsense-mediated mRNA decay, the achilles heel of recurrent glioblastoma?
Vassella group Prof. Dr. Erik Vassella
We conducted CRISPR/CAS interference library screens and identified SMG1, implicated in an evolutionarily conserved RNA quality control pathway - the nonsense-mediated mRNA decay (NMD) pathway. NMD leads to the degradation of transcripts containing premature stop codons, often occurring after temozolomide treatment.
NMD may influence the mechanisms employed by tumour cells to repair DNA damage caused by temozolomide treatment. Hence, we hypothesise that the enhanced temozolomide response is due to reduced DNA repair capacity in SMG1-attenuated glioblastoma cells.
The aim of this work is to further investigate the mechanisms leading to an enhanced temozolomide response in glioblastoma cells with attenuated SMG1. Since NMD efficiently suppresses truncated proteins, which are highly immunogenic, we hypothesise that SMG1 inhibition in temozolomide-resistant, recurrent glioblastoma may elicit tumour inflammation. Hence, we expect that NMD improves the treatment response to immune checkpoint inhibitors. This work is currently supported by the Swiss Cancer League.