Understanding role of Ribonuclease inhibitor (RNH1) in Myelopoiesis and Myeloid Malignancies
Allam group Prof. Dr. phil. nat. Ramanjaneyulu Allam
Our previous investigations have unveiled a crucial function of Ribonuclease inhibitor (RNH1) in regulating homeostatic hematopoiesis. In the absence of RNH1, the balance in hematopoiesis was skewed significantly to favour myelopoiesis at the expense of lymphoid and erythroid lineage cells. It has also been observed from our in-vivo mice studies that the increase in myelopoiesis however, did not culminate in a leukemic transformation.
The ongoing project is aimed at elucidating the mechanism behind the RNH1 mediated exacerbation of myelopoiesis under homeostatic conditions and the implications of genetically modifying RNH1 expression in myeloid malignancies. For this, we are utilizing AML in-vitro (cell lines) and in-vivo (mouse) models along with AML patient derived cells.
Our research so far has highlighted an involvement of RNH1 in the AML pathophysiology and ongoing studies are attempted at discerning a therapeutic potential of targeting RNH1 in myeloid malignancies.
A personalized multi-omics discovery and validation platform for recurrent head and neck squamous cell carcinoma (POLARES)
Giger group Prof. Dr. med. Roland Giger
Diagnostic and therapeutic developments in recent years have improved the prognosis for patients with head and neck squamous cell carcinoma (HNSCC). Despite these developments, a significant proportion of patients relapse after an initial response to standard treatment. Salvage treatment options are limited, and personalized treatment approaches that consider the genomic/epigenetic landscape of the tumor are lacking.
The goal of this research is to establish a center of excellence in HNSCC that bridges the gap between genomic analysis and translation of findings into clinical trials. By establishing a multi-omics discovery and validation platform under the umbrella of the University Comprehensive Cancer Center Inselspital (UCI), this consortium (ORL, Head and Neck Surgery; Medical Oncology; Radiation-Oncology) aims to determine how alterations at the genomic and epigenetic level regulate carcinogenesis, treatment response and resistance in HNSCC and thereby identify novel mechanisms to target tumor relapse.
On behalf of the Consortium: Prof. Dr. Roland Giger (Lead), Otorhinolaryngology, Head and Neck Surgery; PD Dr. Olgun Eliçin, Radio-Oncology; Dr. Simon Häfliger, Medical Oncology; PD Dr. Michaela Medová, Radio-Oncology, DBMR; Prof. Dr. Carsten Riether, Medical Oncology, DBMR; Dr. Daniel H. Schanne, Radio-Oncology
Lung Cancer and Non-Coding RNAs (ncRNAs)
Häfliger group Dr. med. et phil. nat. Simon Häfliger
Lung cancer is the leading cause of cancer related death worldwide. Our research delves into the intriguing world of non-coding RNAs (ncRNAs), RNA molecules that do not translate into proteins.
- MicroRNAs (miRNAs): These tiny RNA molecules can act as either tumor suppressors or oncogenes, influencing cancer development.
- Long Non-Coding RNAs (lncRNAs): These longer RNA molecules impact gene expression and are central to epigenetic regulation.
Using public databases, blood of lung cancer patients, cancer cell lines and bioinformatics tools, we investigate specific ncRNAs with the aim to explore:
- Diagnostic Biomarkers: Can circulating ncRNAs serve as early indicators of lung cancer?
- Mechanisms of Action: How do these ncRNAs influence cancer progression?
- Predictive and Prognostic Markers: Can they guide treatment decisions?
In summary, our work bridges the gap between complex scientific research and practical applications, unraveling the mysteries of ncRNAs in lung cancer to improve patient outcomes and treatment strategies.
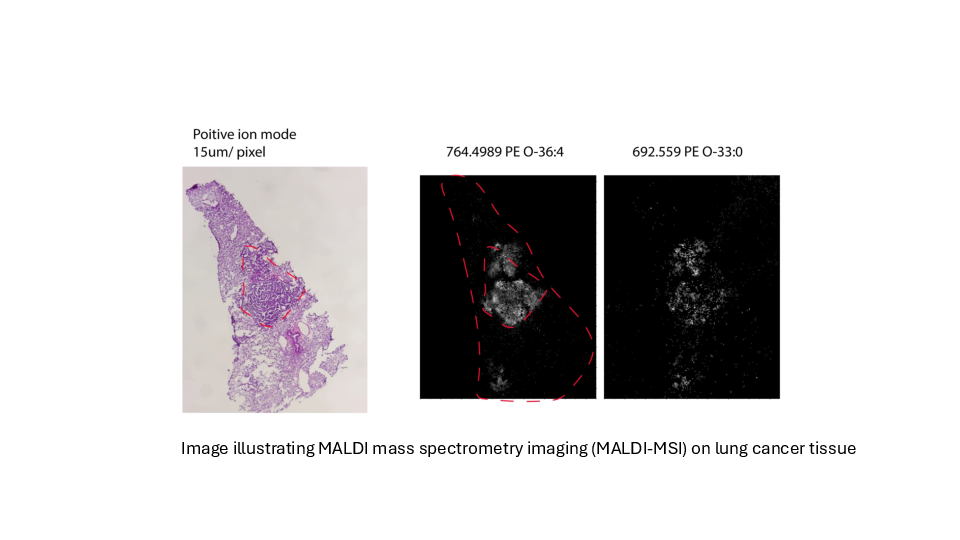
Determing tumor lipid heterogeneity in lung cancer
Konstantinidou group PD. Dr. Georgia Konstantinidou, PhD
Lung cancer is the most common cause of cancer-related deaths worldwide. Tumor-associated mutations of KRAS occur in approximately 30% of non-small cell lung cancer, the most common form of lung cancer.
KRAS mutations are associated with aggressive, metastatic, and treatment-resistant cancers in both humans and mouse models. Mutant KRAS drives a complex network of lipid metabolic rearrangements to help cancer cells adapt to hypoxia and ensure their survival.
We plan to determine the transcriptomic and lipidomic changes that occur in lung tumors during cancer progression and therapy resistance and assess their functional significance, accounting for tumor-to-tumor heterogeneity while preserving the spatial organization of cancer cell populations within the tumor microenvironment.
Dissecting the role of tumor cell heterogeneity in Pancreatic Neuroendocrine Tumor progression
Group Marinoni, Perren, Sadowski PD Dr. Ilaria Marinoni, PhD, Prof. Dr. med. Aurel Perren, PD Dr. Martin Sadowski, PhD
Cancer is a dynamic disease; genetic and epigenetic alterations drive intra-tumoral cell heterogeneity, resulting in the selection of aggressive cell populations capable of driving progression and ultimately metastasis.
Pancreatic neuroendocrine tumours (PanNETs) are tumours that arise from the islets of Langerhans. They exhibit intra-tumoral cell heterogeneity, but it is unclear how this evolves during tumour development and how it contributes to progression.
Our previous data suggest that epigenetic changes are the major drivers of progression and cell heterogeneity in PanNETs. By integrating epigenetic and transcriptomic profiles, we found that cell dedifferentiation and metabolic changes characterise the progression from small PanNETs to more advanced ones.
We are currently investigating the evolution of intra-tumoral heterogeneity of PanNETs through space and time. Specific cell subpopulations identified as driving progression could then be targeted to stop metastasis formation. The identification of targetable pathways that impair metastasis formation will provide a rationale for new treatments.
Targeting cellular metabolism to augment cancer therapy
Marti Group PD Dr. med. Thomas Marti
The aim of this project is to investigate how the nucleotide/lactate metabolism and the DNA damage response machinery are associated with the tumor initiating capacity, the chemotherapy response, and the metastatic capacity of lung and mesothelioma cancer stem cells. In addition, we are exploiting treatment induced cellular adaptations as novel targets for cancer therapy.
The interaction between immune cells and leukemia/cancer stem cells
Ochsenbein group Prof. Adrian Ochsenbein, MD
Our research unit examines the interaction between immune cells and cancer stem cells with a focus on leukemia stem cells. Cancer stem cells are resistant to most of the currently available drugs and are responsible for relapse after successful chemotherapy.
We use state of the art techniques to analyse the molecular interactions between immune cells and cancer stem cells in murine models and in clinical samples from cancer patients.
The aim is to develop improved immunotherapies that specifically target cancer stem cells for different types of cancer, especially in hemato-oncological diseases such as leukemia and multiple myeloma. These novel durgs are tested in in preclinical models and in clinical phase 1 and 2 studies.
Artificial Intelligence for Automated QUality Assurance in RadioTherapy for glioblastoma target volume and organs at risk delineation in clinical trials - AQUA RT
Reyes group Prof. Dr. Mauricio Reyes
In this project we aim to test the hypothesis that AI-based auto-segmentation technologies can be used for an AI-assisted multi-criteria quality assurance assessment in radiation therapy. The proposed multicriteria evaluation model is expected to provide a more objective review than traditional approaches, while at the same time focussing on clinically relevant radiotherapy aspects. The proposed Automated QUality Assurance in RadioTherapy (AQUA-RT) framework has the potential to increase consistency, improve delineation quality and reduce workload for routinely challenging quality assurance procedures.
Leukemia stem cells and the bone marrow microenvironment
Riether group Prof. Carsten Riether, PhD
The bone marrow (BM) microenvironment is a unique cellular architecture which crucially regulates self-renewal and differentiation potential of hematopoietic stem and progenitor cells through cell-cell interaction or the release of soluble mediators.
These evolutionary conserved processes that evolved to protect normal hematopoietic stem cells from elimination and to regulate demand-adapted responses during inflammation are frequently hijacked in cancer and leukemia.
The goal of our research is to understand the molecular and cellular mechanisms how different components of the BM microenvironment such as immune cells and stromal cells affect disease-initiating and -maintaining leukemia stem cells (LSCs) and protect them from immune-mediated elimination.
We take advantage of state-of-the art technologies, well-established chronic and acute myeloid leukemia mouse and patient-derived xenograft models in order strengthen our understanding on LSCs and to translate our findings into human disease.