Perspectives of Targeted Radionuclide Imaging and Therapy of Fibroblast Activation Protein (FAP) in Cancer
Gourni group PD Dr. Eleni Gourni
Tumors develop within a complex microenvironment consisted of diverse cell types surrounded by a matrix rich of proteins, termed tumor stroma. Stroma includes immune cells, fibroblasts and vascular enothelial cells.
Cancer cells rely on extensive support from the stroma to survive, proliferate and invade, thus making stroma an important potential target for anti-cancer therapy. Targeting elements of stroma, may be a useful therapeutic strategy to prevent tumor growth and progression. One of those elements is the fibroblast activation protein (FAP) which is overexpressed on activated fibroblasts on several tumors types.
The current project aims at designing and evaluating novel FAP-specific inhibitors for the generation of radiotracers with the potential to be used for the diagnosis and treatment of FAP-positive tumors. The novel radiotracers are thoroughly investigated in vitro and in vivo using cell lines and xenografted tumor models to understand their binding properties and their in vivo performance.
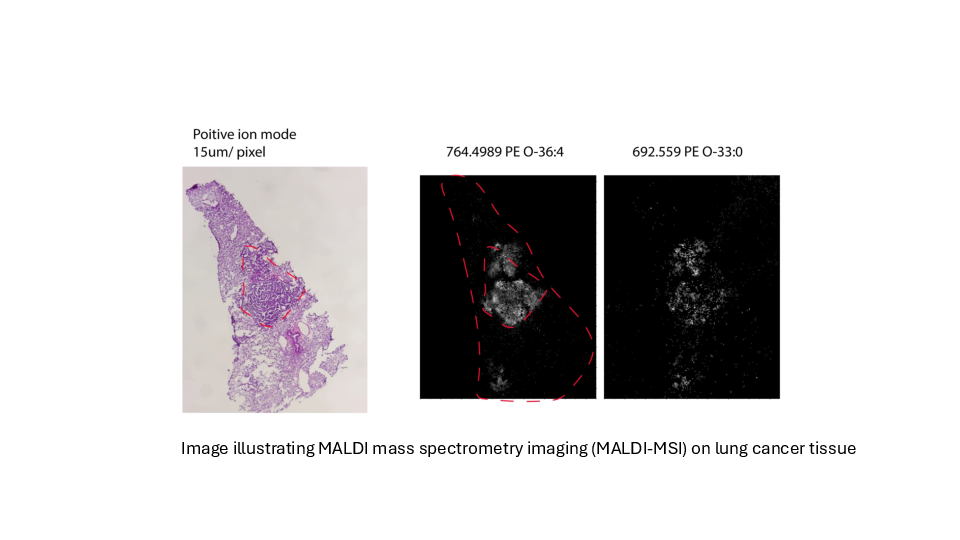
Determing tumor lipid heterogeneity in lung cancer
Guenat group Prof. Dr. Olivier T. Guenat
The Metastasis-on-Chip project aims to replicate the metastatic process, focusing specifically on extravasation and colony formation.
Our initial studies evaluate the metastatic potential of cancer cells based on their phenotypes, using the A549 non-small cell lung cancer (NSCLC) cell line, which exhibits distinct phenotypic variations. We discovered that paraclones, characterized by a mesenchymal phenotype, successfully extravasate, while holoclones, with an epithelial phenotype, do not. Additionally, paraclones demonstrated significantly greater migratory behavior compared to holoclones.
These findings provide valuable insights into the mechanisms of metastasis and lay the groundwork for further exploration of targeted therapies.
Lung Cancer and Non-Coding RNAs (ncRNAs)
Häfliger group Dr. med. et phil. nat. Simon Häfliger
Lung cancer is the leading cause of cancer related death worldwide. Our research delves into the intriguing world of non-coding RNAs (ncRNAs), RNA molecules that do not translate into proteins.
- MicroRNAs (miRNAs): These tiny RNA molecules can act as either tumor suppressors or oncogenes, influencing cancer development.
- Long Non-Coding RNAs (lncRNAs): These longer RNA molecules impact gene expression and are central to epigenetic regulation.
Using public databases, blood of lung cancer patients, cancer cell lines and bioinformatics tools, we investigate specific ncRNAs with the aim to explore:
- Diagnostic Biomarkers: Can circulating ncRNAs serve as early indicators of lung cancer?
- Mechanisms of Action: How do these ncRNAs influence cancer progression?
- Predictive and Prognostic Markers: Can they guide treatment decisions?
In summary, our work bridges the gap between complex scientific research and practical applications, unraveling the mysteries of ncRNAs in lung cancer to improve patient outcomes and treatment strategies.
Determing tumor lipid heterogeneity in lung cancer
Konstantinidou group PD. Dr. Georgia Konstantinidou, PhD
Lung cancer is the most common cause of cancer-related deaths worldwide. Tumor-associated mutations of KRAS occur in approximately 30% of non-small cell lung cancer, the most common form of lung cancer.
KRAS mutations are associated with aggressive, metastatic, and treatment-resistant cancers in both humans and mouse models. Mutant KRAS drives a complex network of lipid metabolic rearrangements to help cancer cells adapt to hypoxia and ensure their survival.
We plan to determine the transcriptomic and lipidomic changes that occur in lung tumors during cancer progression and therapy resistance and assess their functional significance, accounting for tumor-to-tumor heterogeneity while preserving the spatial organization of cancer cell populations within the tumor microenvironment.
Targeting cellular metabolism to augment cancer therapy
Marti Group PD Dr. med. Thomas Marti
The aim of this project is to investigate how the nucleotide/lactate metabolism and the DNA damage response machinery are associated with the tumor initiating capacity, the chemotherapy response, and the metastatic capacity of lung and mesothelioma cancer stem cells. In addition, we are exploiting treatment induced cellular adaptations as novel targets for cancer therapy.
Oncogenic signaling via receptor tyrosine kinases in crosstalk with DNA damage repair
Medová Group PD Dr. med. Michaela Medová
Tyrosine kinase receptors activate a wide range of different cellular signaling pathways. Physiologically, intact signaling via the MET receptor is indispensable in embryonic development and tissue homeostasis. At the same time, MET dysregulation promotes features clearly associated with tumor growth and progression such as uncontrolled proliferation, angiogenesis, local invasion, and systemic dissemination.
Accumulating data suggest that MET signaling may also protect tumor cells from DNA damage, hence relating its aberrant activity to resistance to DNA-damaging agents routinely used in cancer treatment.
We have identified a previously unreported phosphorylation site on MET, which can be recognized by DNA damage master kinases and is involved not only in cellular responses towards DNA damage, but also in metastatic processes, cancer cell migration, and anchorage-independent growth.
This project aims at dissecting the nature, function, and regulation of this phosphorylation site in oncogenic signaling of the receptor.
Unravelling therapy resistance mechanisms for precision medicine in lung cancer and mesothelioma
Peng group Prof. Ren-Wang Peng
Lung cancer and malignant pleural mesothelioma (MPM) are major thoracic tumors characterized by high morbidity and mortality, as well as high heterogeneity and resistance to therapy.
There is an unmet need for a better understanding of the resistance mechanisms, the identification of novel targets and strategies to prevent or overcome therapeutic resistance, and the rational development of precision medicine approaches for personalized disease management to improve clinical outcomes for patients with thoracic tumors.
Towards understanding the role of the minor spliceosome in cancer
Rubin group Prof. Mark A. Rubin MD
Genes are composed of coding units (exons), interspersed with non-coding regions called introns. The process of protein production involves splicing together exons while removing introns from the mRNA molecule. Evolution has given rise to a cellular apparatus called the spliceosome, responsible for carrying out this splicing process.
Alternative splicing enables the generation of diverse protein isoforms from a single gene. Splicing is tightly regulated under normal physiological conditions.
Our recent findings indicate that cancer cells use a specialized spliceosome, the so-called minor spliceosome, to increase cancer relevant mRNAs. As such cancer hijacks the minor intron-splicing machinery to enhance the expression of transcripts containing minor introns. Proteins encoded by those genes have been shown to activate critical cell survival pathways such as cell cycle regulation and DNA repair.
Exploiting the reliance of cancer cells on minor intron-containing genes presents a novel therapeutic opportunity for targeting cancer. By inhibiting the minor spliceosome, we can selectively induce cell death in cancer cells while sparing healthy neighboring cells.